Characterization of (reverse) remodeling in atrial cardiomyocytes using a populations of models approach
- chair:Computational Cardiac Modeling
- type:Bachelor thesis
- tutor:
- person in charge:
Motivation and background
 More than 50 million individuals worldwide are affected by atrial fibrillation (AF), which is the most common sustained arrhythmia and leads to an increased risk of stroke and heart failure. One of the main contributors to AF maintenance is a process called atrial remodeling, which describes structural and electrical changes of single cardiomyocytes and tissue. The standard treatment procedure for patients with AF is the electrical isolation of the pulmonary veins using catheter ablation. In recent clinical trials, additional image- or mapping-guided ablation of remodeled areas in the atrial body was performed, attempting to reduce the recurrence rates after pulmonary vein isolation. However, these procedures could not substantially increase the success rate of recurrence freedom from AF.
More than 50 million individuals worldwide are affected by atrial fibrillation (AF), which is the most common sustained arrhythmia and leads to an increased risk of stroke and heart failure. One of the main contributors to AF maintenance is a process called atrial remodeling, which describes structural and electrical changes of single cardiomyocytes and tissue. The standard treatment procedure for patients with AF is the electrical isolation of the pulmonary veins using catheter ablation. In recent clinical trials, additional image- or mapping-guided ablation of remodeled areas in the atrial body was performed, attempting to reduce the recurrence rates after pulmonary vein isolation. However, these procedures could not substantially increase the success rate of recurrence freedom from AF.
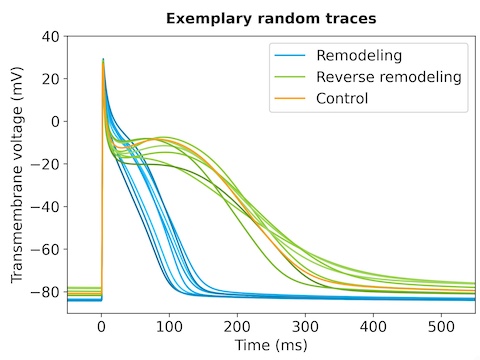
As an alternative to destructing remodeled cardiomyocytes, we investigated the potential to reverse the remodeling process and restore cells to a healthy state. We induced AF in pigs for 4 or 8 weeks and analyzed the remodeling state using action potential and ionic current measurements. We compared the results to study groups that had AF for 4 or 8 weeks followed by sinus rhythm restoration (healthy state) for 4 or 8 weeks. Surprisingly, the cardiomyocytes showed action potentials and ionic currents comparable to those in healthy pigs. This study aims to investigate the mechanisms underlying reverse remodeling and identify the patient populations most likely to benefit.Student project
You will integrate experimental data and single cell simulations to characterize the differences between remodeled and reverse remodeled cardiomyocytes. First, you will identify action potential parameters that best capture the morphological changes observed in the data set. Then, you will perform parameter sampling and optimization of single cell models to achieve representations that closely align with the experimental observations. This approach aims to provide mechanistic insights into the differences between healthy, remodeled, and reverse remodeled cardiomyocytes. Furthermore, utilizing these intrinsic restoration processes to repair remodeled cells may represent a novel alternative to their destruction using catheter ablation.

